

 |
 |
How Do We Know Any of This? Rutherford's Analysis 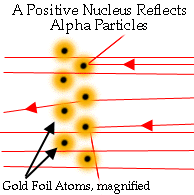
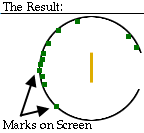
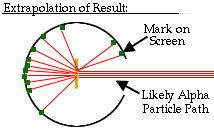
Since some of the positive alpha particles were substantially deflected, Rutherford concluded that there must be something inside an atom for the alpha particles to bounce off of that is small, dense, and positively charged: the nucleus.
|